Fill in Your Control Substance Inventory Michigan Form
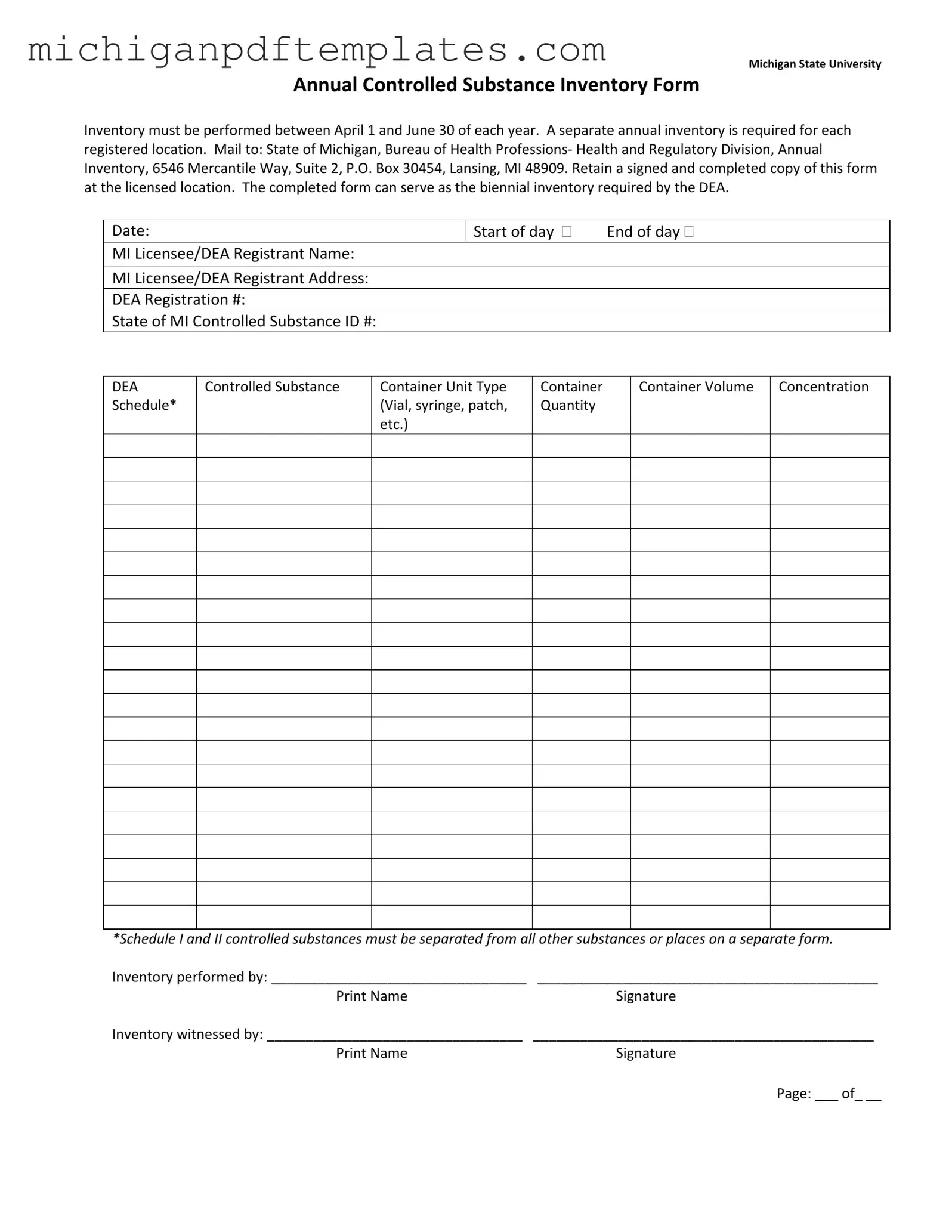
The Control Substance Inventory Michigan form is a crucial document required for tracking controlled substances at registered locations within the state. This annual inventory must be conducted between April 1 and June 30 each year, ensuring compliance with state regulations. Each location must maintain a signed and completed copy of the form, which can also fulfill the biennial inventory requirement mandated by the DEA.
To complete the form, click the button below.
Get Your Form Now
Fill in Your Control Substance Inventory Michigan Form
Get Your Form Now

Get Your Form Now
or
▼ PDF Form
Finish this form quickly and move on
Fill in and complete Control Substance Inventory Michigan online quickly.